Free Shipping For Orders Over 100 Riyal
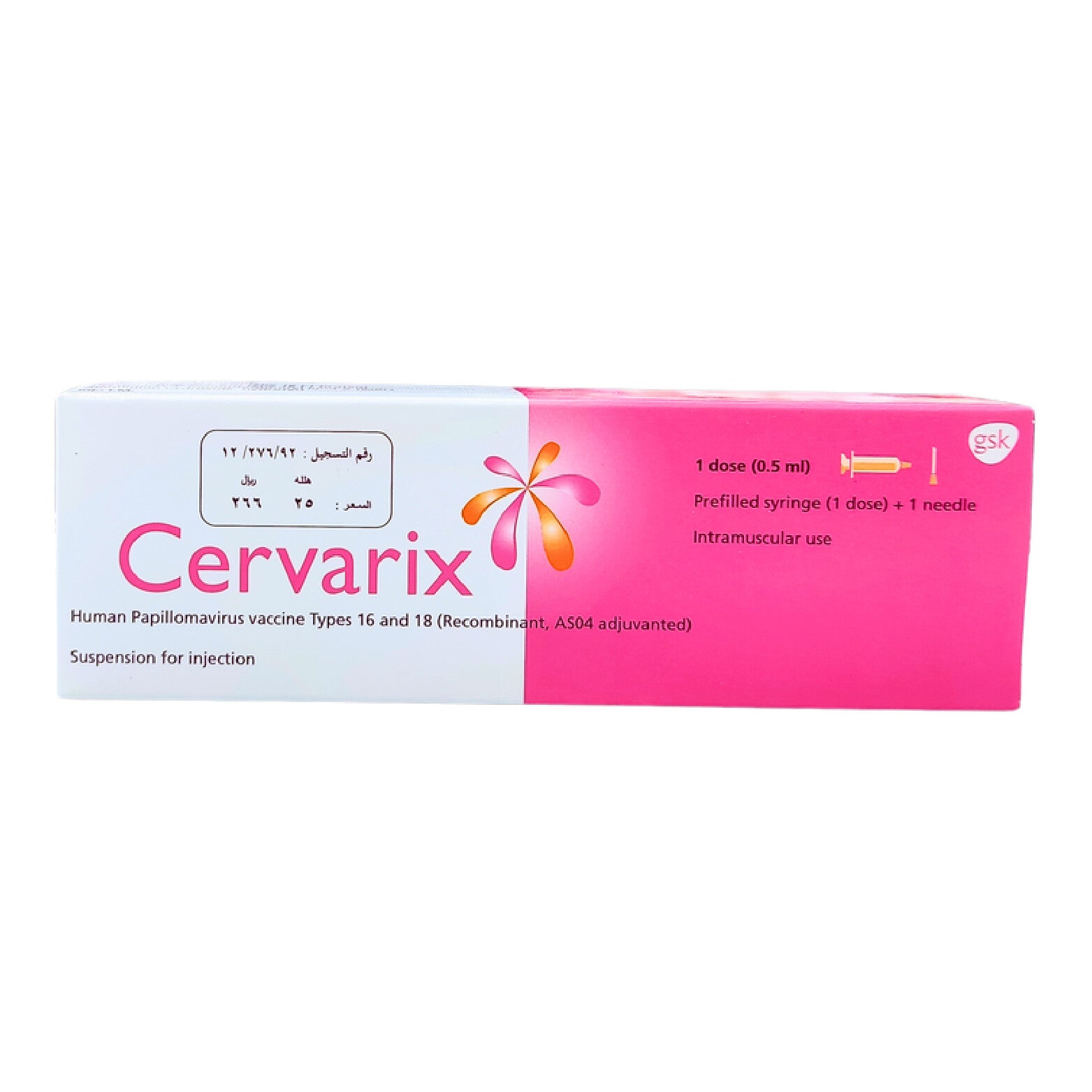
Cervarix Vaccine 1Dose .5Ml
-
266.25 SR

-

-
266.25

Couldn't load pickup availability
Description
xCervarix Vaccine 1Dose 0.5Ml provides protection against human papillomavirus (HPV), which causes cervical cancer in women.
Indications for Use of Cervarix 0.5 ml
Cervarix is a vaccine used to prevent cervical cancer. It can be administered to females from the age of 9 years to protect against HPV-related cancers.
Dosage and Administration of Cervarix vaccine
-
Ages 9–14 years: Two doses of 0.5 ml each. The second dose is given 5–13 months after the first dose.
-
Ages 15 years and older: Three doses of 0.5 ml each. The second dose is given 1 month after the first dose, and the third dose 6 months after the first dose.
-
Cervarix is administered via intramuscular injection into the deltoid muscle (upper arm).
Side Effects of Cervarix
Common side effects may include:
-
Headache
-
Dizziness
-
Gastrointestinal disturbances such as nausea, vomiting, or diarrhea
-
Itching
-
Skin rash
-
Joint pain
Contraindications
Do not use Cervarix vaccine in cases of hypersensitivity to any of its components.
Warnings and Precautions
-
Fainting may occur after vaccination, particularly in adolescents, as a psychological response to needle injection.
-
The vaccine protects only against HPV types 16 and 18.
-
Cervarix is preventive; it does not treat existing HPV infection or cancers caused by HPV.
-
Avoid use during pregnancy or when planning to become pregnant.
-
Consult a doctor before receiving Cervarix during breastfeeding.
Storage Instructions
-
Store in a refrigerator at 2–8°C.
-
Do not freeze.
-
Keep in the original packaging, protected from light.
-
Use immediately after opening. If not used right away, keep refrigerated. Discard if not used within 6 hours.
Shippping & Return
xسياسة الاسترجاع والاستبدال
هدفنا في متجر الجواهر هو رضاكم التام 🌸، ونسعى دايمًا نقدم أفضل تجربة تسوق بكل وضوح وعدل. نتعامل مع عملائنا بكل مصداقية، ونتمنى يكون التعامل متبادل بنفس الروح.
في حال تم طلب الإلغاء أو الاسترجاع قبل بدء الشحن، يتم إرجاع المبلغ كامل للعميل.
ويحق للعميل طلب الاسترجاع خلال مدة 3 أيام من تاريخ الاستلام، بشرط:
- أن يكون المنتج غير مستخدم ولم يتم فتحه.
- أن يكون بحالته الأصلية وخالي من أي تلف.
- ألا يكون المنتج من الأصناف المستثناة من الاسترجاع أو الاستبدال.
المنتجات غير القابلة للاسترجاع أو الاستبدال:
- المنتجات التي تحتاج تخزين خاص مثل: أدوية الثلاجة، الحليب، وأغذية الأطفال.
- مزيلات العرق ومنتجات العناية الشخصية والنسائية، إلا في حال وجود عيب مصنعي.
طريقة طلب الاسترجاع أو الاستبدال:
للطلب، يرجى التواصل معنا عبر واتساب على الرقم:
0563649160
في حال طلب الاسترجاع:
بعد التأكيد، يتم إرسال بوليصة شحن للعميل لتسليم المنتج لشركة الشحن وهو بحالته الأصلية.
بعد استلام شركة الشحن للمنتج، يتم إرجاع المبلغ بعد خصم قيمة الشحن خلال مدة من 5 إلى 7 أيام عمل.
في حال طلب الاستبدال:
يتم احتساب قيمة شحن جديدة للطلب البديل.
تنويه مهم:
في حال وصول المنتج بشكل خاطئ من شركة الشحن (باستثناء أدوية الثلاجة، الحليب، وأغذية الأطفال) أو تم إرسال منتج غير صحيح من صيدلية الجواهر المميز، يرجى التواصل مع خدمة العملاء، وبعد التأكد من المشكلة سيتم إرجاع المبلغ كاملًا بعد تسليم المنتج لشركة الشحن.
في حال وصول المنتج مفتوح أو مستخدم أو بحالة غير التي تم شحنه بها، سيتم إلغاء طلب الاسترجاع.
في حال كان الخطأ من شركة الشحن أو تم إرسال منتج خاطئ، نتحمل تكلفة إرسال المنتج لفرع شركة الشحن عبر تطبيقات التوصيل بحد أقصى 20 ريال، في حال كان الفرع بعيد.
في حال عدم استلام العميل للطلب وعودة الشحنة لنا، يتم إرجاع المبلغ بعد خصم قيمة الشحن، وإذا رغب العميل بإعادة الشحن مرة أخرى يتم دفع قيمة شحن جديدة.
You may also like!
Recently Viewed Products
- Choosing a selection results in a full page refresh.



